The definition of detergent/s are product/s intended for use of washing and cleaning process in domestic or industrial areas (examples as in washing machines; dish washers etc). Detergents are surfactants which reduce the surface tension of water when used in very low concentrations. The products used to prewash or whiten, fabric softeners or intended for cleaning purposes are classified as detergents. Detergency is the process of cleaning where a detergent removes contaminants from a surface by solubilizing, suspending, or emulsifing them. A detergent does not allow the contaminant from reabsorbing on the surface. The process of detergency involves three phases: surface/soil/detergent and several physicochemical phenomenon taking place at these interface. First is wetting of the surface, second is the removal of soil from surface by solubilizing, suspending, or emulsifing them and third involves avoiding the re-deposition of soil on surface..

For effective detergent action, it is necessary to understand the types of soil, eg proteins, lipids, sugars, or mineral based.In reality soils can be more complex as mixtures of sugar + exopolysaccharides +glycoproteins + lipopolysaccharides; or lipids + lipopolysaccharides +lipoproteins; or proteins + glycoproteins +lipoproteins; or limestone or dust and other particles (metals like iron, copper etc….). And secondly, the soil state and whether mechanical actionwill be necessary for removal. The HLB (hydrophilic-lipophilic balance) of detergent determines the best detergent for each type of soil and its effective removal. Low HLB indicates, lack of affinity for water and more for oils/lipophilic soils, while high HLB indicates vice-versa, higher affinity for water phase/water soluble soils and lack of affinity for oil/fat based substances. Certain times the higher pH (>9) of soaps or detergents enables the saponification (hydrolysis) of oils/fats and rinse with water.
Detergent cleaning action for surfaces is a comparatively mild cleaning technique. The detergent surrounds the dirt particles, taking them into suspension without actually dissolving the material. This action is assisted by wetting agents and surfactants that loosen the particles from the surface. Dishwash liquid soap is an excellent detergent for many applications such as cleaning surfaces. Metal ions, such as the calcium and magnesium, which are found in hard water, make the soaps insoluble thus leaving a residue and this is a major problem with soap formulations. Chelating agents are hence added to prevent the formation of residues.
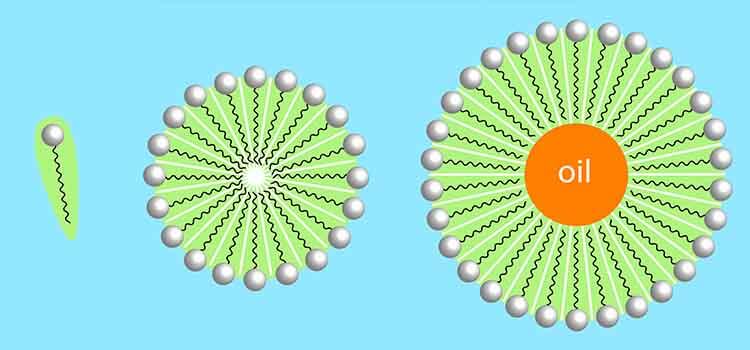
detergents are classified as amphiphilic molecules, since they contain hydrophobic and hydrophilic parts in their structures. The hydrophobic group usually consists of a hydrocarbon tail derived from fatty alcohol, acids or amines while the hydrophilic part has a polar head derived from ethoxylate or propoxylate or a combination of both. The hydrophobic part is more soluble in organic solvents or oils while the hydrophilic portion is soluble in aqueous solutions. Due to their dual (amphiphilic) nature, these chemicals migrate to the interface(s) when placed in solution and their alignment at interfaces reflects their tendency to assume the most energetically favoured orientation.detergents, when added at the appropriate concentrations, in aqueous environment, are capable of forming micelles. This concentration which allows formation of micelle in water is called critical micelle concentration (CMC) and is an important factor during detergent action andsolubilization. The formation of micelles is a consequence of the dual nature of detergents and not only depends on the detergent's chemistry but also on the temperature, the pH and the ionic strength of the solution.The CMC concentration of detergent can be determined empirically by physical measurements, eg, surface tension, or by chemical methods using addition of dyes changing the color while micelles are formed.
The three main groups of detergents are classified into nonionic, ionic and zwitterionic. Nonionic detergents, as discussed earlier are amphiphilic molecules, containing hydrophobic and hydrophilic parts in their structures. When they are dissolved in water, they do not have charge in the area of the hydrophilic head. These are effective in breaking interactions between lipids or lipids and proteins. Some examples are tritons (X-100, X-114), SPANs, NP-40 or tweens. Ionic detergents possess a stable charge located on the hydrophilic part, when dissolved in water solutions. Sodium dodecyl sulfate (SDS), is one of the most widely used detergents of this group. The last group, zwitterionic detergents, are the substances simultaneously possessing positive and negative charges but their net charge is equal to zero. Zwitterionic detergents like sulatines, betaines, do not change their own charge while solubilizing proteins. They are also more useful for solubilisation of the proteins than non-ionic detergents, mainly due to the inhibition of protein–protein aggregation.
Detergents have an important factor or phenomenon called the “cloud point” (CP). The name of this phenomenon is taken from the “clouds” formed in the detergent solution after reaching its cloud point temperature (CPT). This phenomenon depends on the temperature of the medium in which detergent is dissolved. The solution separates into two phases: (1) the aqueous part, and (2) the “cloudy” part containing detergent molecules. CP is mainly observed for nonionic detergents, but others (eg, SDS) have also their own cloud points at higher temperatures, usually below 100°C. Some detergents like Triton X-114 have relatively low cloud point temperatures (23°C).
