The compound that lower the surface tension between two phases, like between two liquids, a gas and a liquid, or a liquid and a solid are called surface active agent or surfactant..

In aqueous solutions surfactants behave like organic compounds that are amphiphilic, meaning they contain both hydrophobic groups (their tails) and hydrophilic groups (their heads). The arrangement of the hydrophilic head is at the interface of water and the hydrophobic groups aligns toward oil. Therefore, a surfactant contains both a water-insoluble (or oil-soluble) component and a water-soluble component. Surfactants will diffuse in water and adsorb at interfaces between air and water or at the interface between oil and water, in the case where water is blended with oil. This property allows surfactants to act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants.
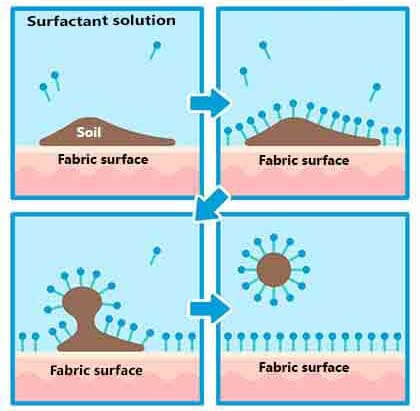
Fig. 1.1 - Surfactant/detergent in action; top left panel- surfactant molecules in solution with soil on the fabric surface. Topright-surfactant consist of hydrophilic and lipophilic portions which align with the type of dirt/soil. Bottom left-Surfactant adsorb on surface of soil/dirt and start the process of solubilizing, or emulsifing them. Bottom right- Surfactant molecules envelop the dirt/soil molecules preventing them from reabsorbing to the surface giving the cleaning action.
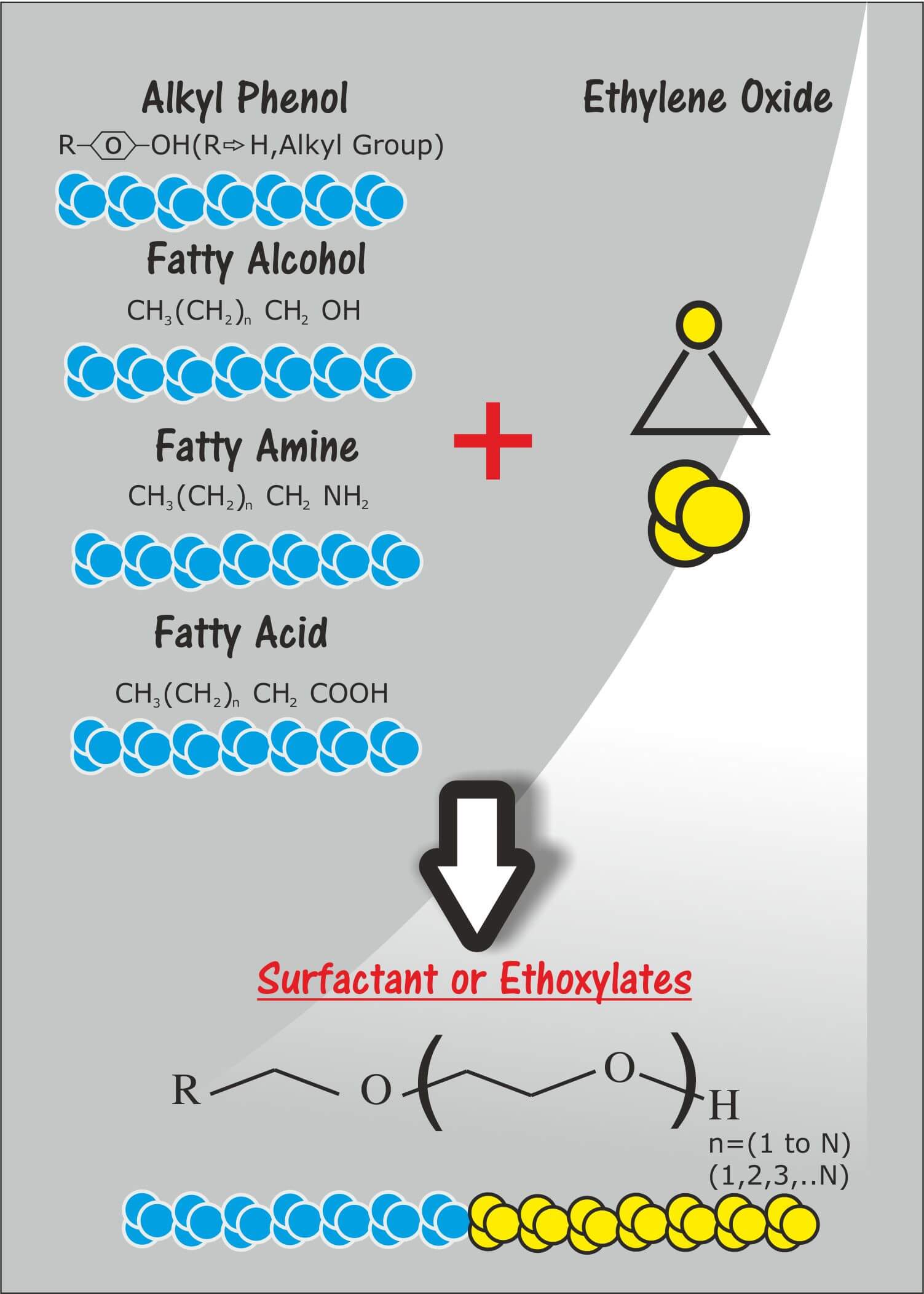
Many important surfactants include a polyether chain terminating in a polar anionic group. The polyether groups often comprise ethoxylated (polyethylene oxide-like) sequences inserted to increase the hydrophilic character of a surfactant. Polypropylene oxides conversely, may be inserted to increase the lipophilic character of a surfactant. Surfactant molecules have either one tail or two; those with two tails are said to be double-chained. Surfactant classification according to the composition of their head falls under nonionic, anionic, cationic, and amphoteric. A nonionic surfactant has no charged groups in its head. The head of an ionic surfactant carries a net positive, or negative charge. If the charge is negative, the surfactant is more specifically called anionic; if the charge is positive, it is called cationic. If a surfactant contains a head with two oppositely charged groups, it is termed zwitterionic. Zwitterionic or amphoteric surfactants have both cationic and anionic centers attached to the same molecule.
Two major surfactants, are linear alkylbenzene sulfonates (LAS) and the alkyl phenol ethoxylates (APE). anionic surfactants contain anionic functional groups at their head, such as sulfate, sulfonate, phosphate, and carboxylates. Prominent alkyl sulfates include sodium lauryl sulfate (sodium dodecyl sulfate, SLS, or SDS), and the related alkyl-ether sulfates sodium laureth sulfate (sodium lauryl ether sulfate or SLES), and sodium myreth sulfate.
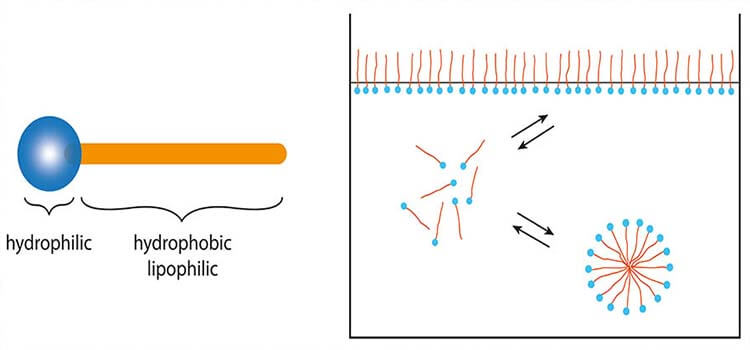
Fig. 1.2 - Surfactant in action.
Nonionic surfactants have covalently bonded oxygen-containing hydrophilic groups, which are bonded to hydrophobic parent structures. The water-solubility of the oxygen groups is the result of hydrogen bonding. Hydrogen bonding decreases with increasing temperature, and the water solubility of nonionic surfactants therefore decreases with increasing temperature. The characteristic feature of cloud point (CP) of nonionic surfactants is the temperature at which the surfactant separates out from an aqueous solution due to the weakening of hydrogen bonds between the surfactant and water molecules. Nonionic surfactants are less sensitive to water hardness than anionic surfactants, and they foam less strongly.
Most anionic and nonionic surfactants are nontoxic, having LD50 comparable to sodium chloride. The toxicity of quaternary ammonium compounds, which are antibacterial and antifungal, varies. Prolonged exposure to surfactants can irritate and damage the skin because surfactants disrupt the lipid membrane that protects skin and other cells. Skin irritancy generally increases in the series nonionic, amphoteric, anionic, cationic surfactants..
Application
Surfactants play an important role in personal care products such as cosmetics, shampoos, shower gel, hair conditioners, and toothpastes. The property of providing cleaning, wetting, and dispersing, emulsifying, foaming and anti-foaming effects are used in many practical applications and products. Surfactants with different HLB are used in detergents, fabric softeners, soaps, paints, adhesives, inks, emulsions, anti-fogs, ski waxes, snowboard wax, deinking of recycled papers, in flotation, washing and enzymatic processes. Agrochemical formulations such as some herbicides, insecticides, biocides (sanitizers), and spermicides also contain Surfactants. Surfactants find use in firefighting and pipelines as liquid drag reducing agents. Alkali Surfactant polymers are used to mobilize oil in oil wells for exploration.
Types of surfactants
Nonionic surfactant refers to the surfactant molecules, which do not undergo ionization when being dissolved in water. The Nonionic surfactant are not in the ionic state in the solution, thereby having high stability and being less susceptible to the effect of strong electrolyte inorganic salts as well as acid and alkalis. Nonionic surfactants have excellent compatibility with other types of surfactants and have excellent solubility (which vary depending on different structures, HLB etc) in both water and organic solvents.
Nonionic surfactants have covalently bonded oxygen-containing hydrophilic groups, which are bonded to hydrophobic parent structures. These Nonionic surfactants, are not ionized in water, and contain both hydrophilic groups (e.g. oxyethylene-CH2CH2O-, ether groups, hydroxyl group -OH or -CONH2 amide group, etc.) and lipophilic group (e.g., hydrocarbons which can be natural fatty alcohols or synthetic alcohols, acids or glyceryl esters/oils). The water-solubility of the oxygen groups is the result of hydrogen bonding. Hydrogen bonding decreases with increasing temperature, and the water solubility of Nonionic surfactants therefore decreases with increasing temperature. This result in formation of a milky/cloudy emulsion called the cloud point of surfactants. This property is very essential for determining the optimum use of Nonionic surfactant in formulations at elevated temperature especially in cleaning formulations like detergents, CIP etc.
As discussed above Nonionic surfactants have a unique property called a cloud point. The cloud point is the temperature at which the Nonionic surfactant begins to separate from the cleaning solution, called phase separation. When this occurs, the cleaning solution becomes cloudy. This cloud point is therefore considered the temperature for optimal detergency. For low foaming cleaners, optimal detergency is at the cloud point; for foaming cleaners optimal detergency is either just below the cloud point or at the start of the cloud point. The agitation of low foaming cleaners is sufficient to prevent phase separation. The temperature of the cloud point depends upon the ratio of the hydrophobic and hydrophilic portions of the Nonionic surfactant. Some cloud points are at room temperature while others are very high. Some Nonionic surfactants don’t have a cloud point because they have a very high ratio of hydrophilic to hydrophobic moieties.
Nonionic surfactants are less sensitive to water hardness than anionic surfactants, and they foam less strongly. The differences between the individual types of Nonionic surfactants are slight, and the choice is primarily governed having regard to the costs of special properties (e.g., effectiveness and efficiency, toxicity, dermatological compatibility, biodegradability) or permission for use in food. In areas with hard water (high mineral content), Nonionic surfactants are more heavily marketed, as they are less likely to form a soap scum. The Nonionic surfactants are less likely to cause skin irritation, but this is associated with a less potent cleaning ability. Most cleaning products are manufactured as a blend of anionic and Nonionic surfactants to balance out the cleaning potential with the risk of skin irritation.
The aqueous solution of Nonionic surfactants has poor foaming capability with the foam being not stable as well. This is due to that each molecule of the Nonionic surfactant has relatively large surface area and the interface being in uncharged foam. Polyoxyethylene has long chain and uniform molecular weight distribution. The lipophilic group has long chain and also contains branched chain. The presence of the polyoxyethylene -polyoxypropylene copolymer both has a great impact on the foaming of the Nonionic surfactants. Owing to the presence of the polar portion and non-polar portions existing in their molecular structure, they have large surface activity. Such kind of active agents can be divided into the ester type (e.g. polyoxyethylene fatty acid esters, sorbitan fatty acid esters anhydrides), ether type (e.g., polyoxyethylene alkyl ether, polyoxyethylene alkyl phenol ether), amine type (such as polyoxyethylene fatty amine), amide type (such as polyoxyethylene alkyl amide) and mixing type (such as sorbitol anhydride fatty acid esters, polyoxyethylene ether). In the field of oiling, Nonionic surfactants are mainly used in foaming, emulsifying, anti-wax, anti-corrosion, retarder, production increase of oil wells, intensified injection of injection wells as well as improving oil recovery and so on.
| Trade/Common name | Name | Applications |
|---|---|---|
| Product-35-AJ | Polyoxyethylene glycol octylphenol ethers: C8H17–(C6H4)–(O-C2H4)1–25–OH | Wetting agent – coatings |
| Product-30-AI | Polyoxyethylene glycol alkylphenol ethers: C9H19–(C6H4)–(O-C2H4)1–25–OH | Spermacide |
| Polysorbates | Polyoxyethylene glycol sorbitan alkyl esters | Food ingredient |
| Span® | Sorbitan alkyl esters | Polishes, cleaners, fragrance carriers |
| PEGs, PPGs Product L-61, L-62, F-108 | Block copolymers of polyethylene glycol and polypropylene glycol | Various |
Anionic surfactants contain anionic functional groups at their head, such as sulfonate, phosphate, sulfate and carboxylates. Alkyl sulfates include sodium lauryl and the related alkyl-ether sulfates sodium laureth sulfate, also known as sodium lauryl ether sulfate (SLES), and sodium myreth sulfate. Sodium stearate is a good example of a surfactant. It is the most common surfactant in soap. The stearates comprise >50% of the global usage of surfactants. Many of these find utilization in emulsion polymerization. Other anionic surfactants include dioctyl sodium sulfosuccinate (DOSS), linear alkylbenzene sulfonates (LABs) and alkyl-aryl ether phosphates.
| Abbreviation | Name | Applications |
|---|---|---|
| DOSS | Dioctyl sodium sulfosuccinate (DOSS) | Wetting agent – coatings, toothpaste |
| LABSA | Linear alkylbenzene sulfonates | Laundry detergents, dishwasher detergents |
| SLES | Sodium lauryl ether sulfate | Shampoos, bath products |
| N/A | Sodium stearate | Handsoap, HI&I products |
Cationic surfactants are comprised of a positively charged head. Most of cationic surfactants find use as anti-microbials, anti-fungals, etc. in HI&I (Benzalkonium chloride (BKC-80 and BKC-50). The cationic nature of these surfactants disrupts the cell membranes of bacteria and viruses.
Zwitterionic (amphoteric) surfactants have both cationic and anionic centers attached to the same molecule. The anionic part can be variable and include sulfonates, as in the sultaines (Foamer HS). Betaines such as cocamidopropyl betaine (CAPB, coco betaine) have a carboxylate with the ammonium. The cationic part is based on primary, secondary, or tertiary amines or quaternary ammonium cations. Zwitterionic surfactants are often sensitive to pH and will behave as anionic or cationic based on pH. Fast dry (“coacervation”) latex traffic paints are based on this concept, with a drop in pH triggering the latex in the paint to coagulate.
